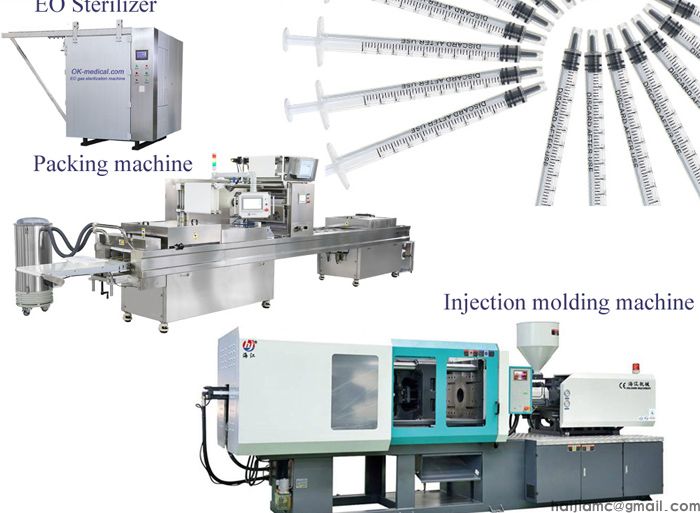
Insulin syringes are medical devices that are used to inject insulin into the body. They are typically made from plastic and have a small, fine needle that is used to deliver the insulin. Here is a general outline of the steps involved in the production of insulin syringes:
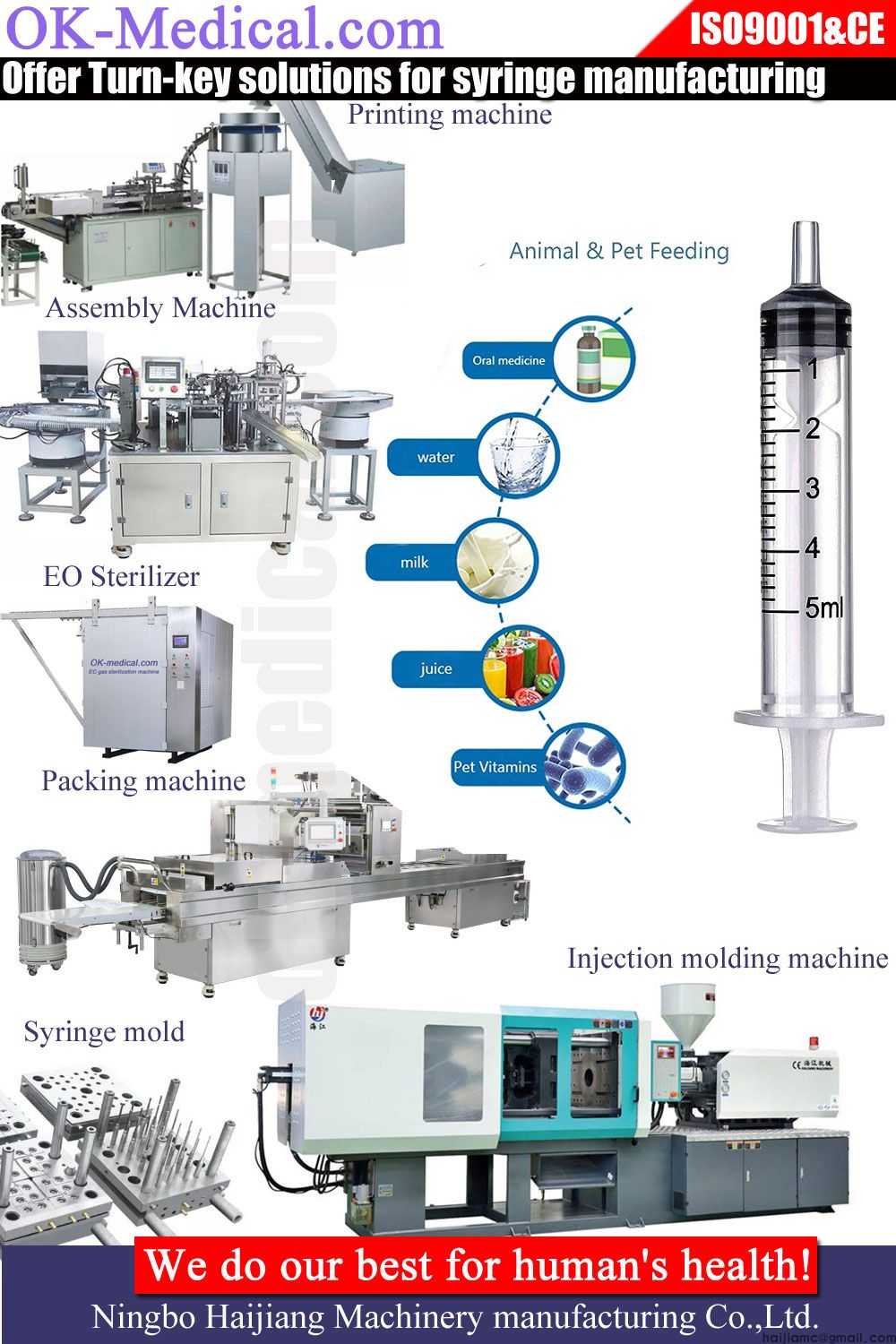
Design and development: The first step in the production of insulin syringes is the design and development of the syringe. This involves creating a detailed design of the syringe, including its size, shape, and features, as well as testing and refining the design to ensure that it meets the necessary performance and safety standards.
Manufacturing the components: Once the design of the insulin syringe has been finalized, the individual components of the syringe are manufactured. This typically involves injection molding the plastic parts, such as the barrel and plunger, and forming the needle from stainless steel wire.
Assembling the syringe: The individual components of the insulin syringe are then assembled into the finished product. This typically involves attaching the needle to the barrel, inserting the plunger into the barrel, and attaching the cap to the plunger.
Quality control: The finished insulin syringes are then subjected to a series of quality control checks to ensure that they meet the required standards. This may include visual inspections, dimensional checks, and functional testing.
Packaging and distribution: Once the insulin syringes have passed quality control, they are packaged and shipped to hospitals, pharmacies, and other distribution points for use by patients.
It is important to follow strict guidelines and regulations in the production of insulin syringes to ensure that they meet the necessary safety and performance standards. This includes using high-quality materials and adhering to good manufacturing practices to ensure the safety and efficacy of the finished product.